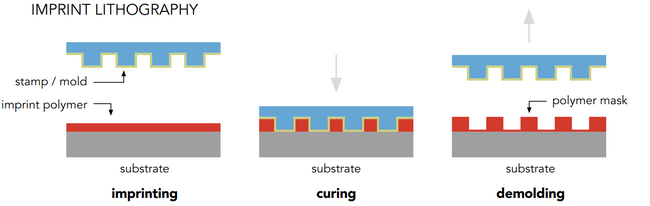
Nanoimprint Lithography and Nanotransfer Printing
Lateral silicon oxide/gold interfaces enhance the rate of electrochemical hydrogen evolution reaction in alkaline media
Thomas L. Maier, Matthias Golibrzuch, Simon Mendisch, Werner Schindler, Markus Becherer, Katharina Krischer
Featured on the Cover Page of J. Chem. Phys. 152, 154705 (2020) AIP Publishing 2020-04;
https://doi.org/10.1063/5.0003295
Nanoimprinting allows us to pattern macroscopic large areas up to 10 x 10 mm with meso- or nanoscopic structures. Once an imprinting mold is fabricated with electron- or ion-beam technologies, the pattern can be transferred on a vast variety of surfaces in a rather cheap and quick process. Replication of the imprinting molds and tuning of feature sizes allows us to vary the properties of each individual sample. With our existing imprinting molds, we can fabricate up to 3 billion identical structures in a defined array. The precision of the nanoimprinting technique in our process is within a few nanometers.
We are part of the IGSSE project team “12.02 Photoelectrochemical CO2 reduction with tailored nanostructured metal/semiconductor electrodes (CO2-NanoCat)”. In the project, we use lift-off nanoimprinting to decorate silicon-based electrodes with gold nanoislands. In cooperation with the physics department, we use such electrodes for (photo-)electrochemical devices to improve overall catalytic performance of silicon-based electrodes.
In the IGSSE project, we investigate the influence of the electrode properties on the electrochemical reactions. Here, the size of the metal nanoislands, their plasmonic resonance, and the combination of substrate and electrode material are of special interest.
Regarding the size of the metal nanoislands, we can produce nanoisland arrays in five different sizes: we can fabricate features sizes of 45 nm, 75 nm, 200 nm, 350 nm, and 1400 nm. All nanoisland arrays have pitches in the area of twice the feature size. Furthermore, we can tune the feature sizes during the process at a constant pitch. The feature sizes can be increased directly in the fabrication process and deceased using a special stamp replication technique. The different base feature sizes and the tuning of the feature sizes enables us to investigate the influence of the metal nanoisland size on the catalytic reactions over almost three orders of magnitude.
The plasmonic properties of the metal nanoisland arrays are also dependent on the feature sizes and the pitch of the islands. With the possibility of tuning the sizes we are able to shift the plasmonic resonance of our electrodes into a desired wavelength region. Furthermore, the flexibility of the substrate allows us to fabricate metal nanoisland arrays on transparent substrate to analyze the change of the plasmonic properties in a transmission measurement. In addition, we investigate the influence of the substrate on the plasmonic properties.
Besides the influence on the plasmonic properties, the substrate influences the electrochemical reactions as well. To suppress the influence of reactions taking place at the silicon electrolyte interface, we developed a technique to embed the metal nanoislands in silicon oxide. Hence, the metal nanoislands are still electrically connected to the silicon substrate, however, the residual surface of the substrate Is passivated by the silicon oxide. Furthermore, the presence of the silicon oxide influences the reactions taking place on the metal electrolyte interface. This bicatalytic effect enhances the performance of our electrodes in electrochemical water splitting.
Funding and Duration
This project is funded by the International Graduate School of Science and Engineering (IGSSE),
Duration: 2018-2021
Cooperation Partners
Technische Universität München TUM
Chair of Nanoelectronics
Chair of Chemical Physics Beyond Equilibrium
Further Information
Principal Investigator: Markus Becherer, Katharina Krischer
Doctoral Candidates: Simon Mendisch, Matthias Golibrzuch
Homepage
https://www.igsse.gs.tum.de/research/project-teams/12th-cohort/1202-co2-nanocat/